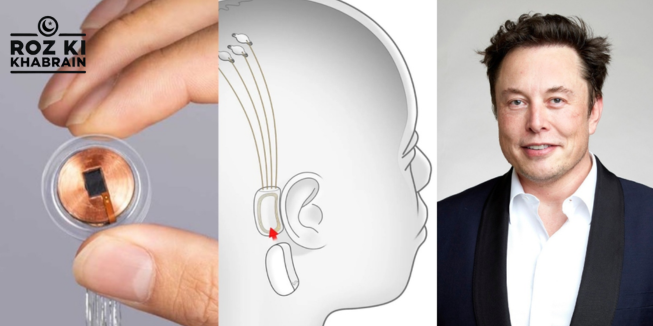
Neuralink Corp, the brain-computer interface company founded by billionaire Elon Musk, has successfully implanted its device in a third patient and plans to expand its experimental procedure by adding 20 to 30 more implants in the coming year.
Musk celebrated this milestone during a Las Vegas event, which was streamed live on his social media platform, X (formerly Twitter). “We now have three humans with Neuralinks implanted, and they’re all working well,” Musk said, emphasizing the progress made in the company’s efforts to merge human cognition with cutting-edge technology.
Advancing Brain-Computer Interfaces
Neuralink is part of a growing sector of startups working on brain-computer interface (BCI) technology, aiming to create devices that could assist individuals with severe neurological conditions, such as paralysis and Amyotrophic Lateral Sclerosis (ALS).
These implants involve complex surgeries to insert electrodes into brain tissue, enabling communication between the brain and external devices. The company began its human trials last year, with its first patient, Noland Arbaugh, successfully receiving the implant. Neuralink’s flagship study, known as the Prime Study, has been approved by the U.S. Food and Drug Administration (FDA) and involves five paralyzed patients who can control external devices, like computers and smartphones, using only their thoughts. Another FDA-approved study, Convoy, focuses on enabling three patients to operate assistive robotic arms, showcasing the potential to help people regain some independence.
Scaling Up in 2025
Musk acknowledged that the technology is still in its early stages, but initial results have been promising. The planned expansion of 20 to 30 additional implants in 2025 marks a significant leap toward broader clinical use. This expansion highlights Neuralink’s goal to revolutionize neural engineering, despite facing challenges such as ethical, technical, and regulatory issues.
While Neuralink is leading in this field, it faces strong competition from other brain-computer interface companies. However, its FDA approvals and ongoing trials position it at the forefront of innovation in this developing area of medicine and technology.
Despite the excitement surrounding Neuralink’s advancements, the company has faced scrutiny over the safety of its procedures and the potential risks of integrating these devices with the human brain. The experimental nature of these implants raises concerns about long-term effects, patient safety, and ethical considerations. Experts believe the success of these trials could lead to broader applications in medical rehabilitation and cognitive enhancement, but they stress that careful research and strict oversight are crucial to ensuring the safety and effectiveness of these technologies.